通过光驱动硝化实现甲烷转化
Published Time: 2023-07-20 14:40:19
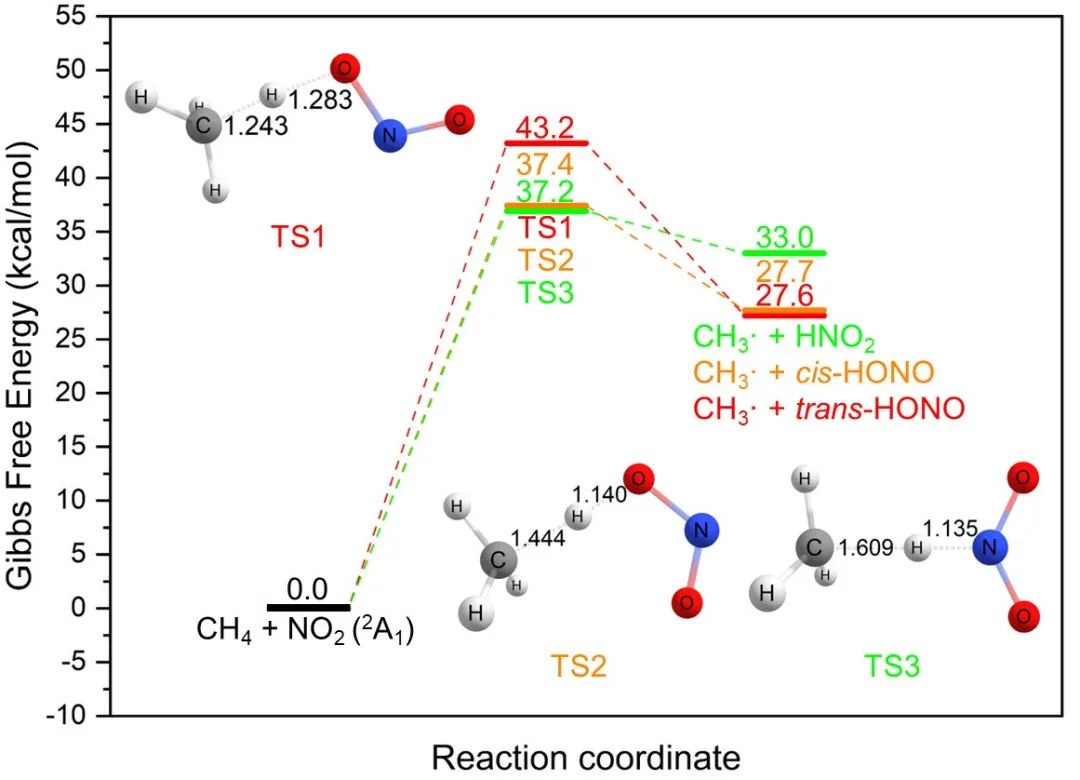
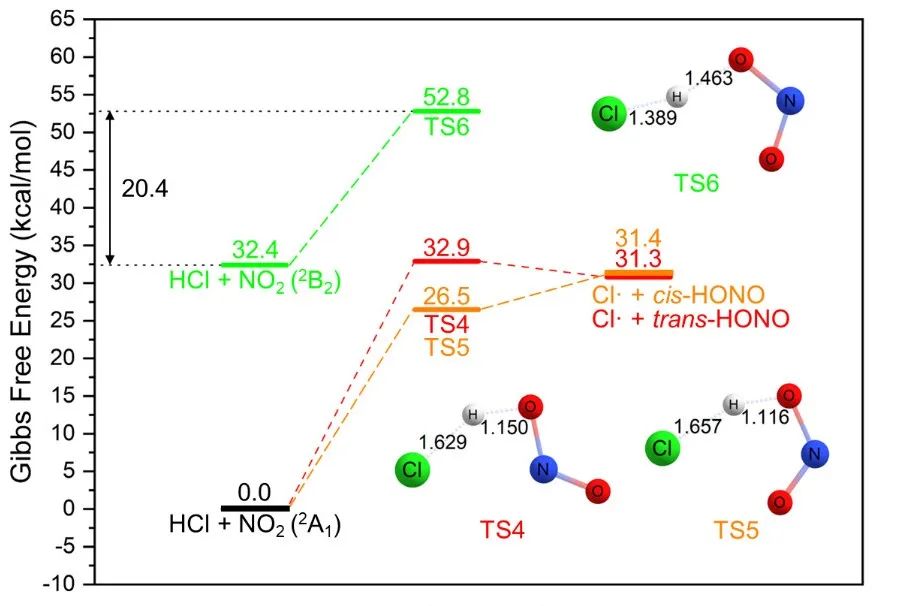
甲烷的活化及其高效转化是一个重要的科学问题。在最近与厦大化院汪骋课题组合作研究中,我们介绍了一种利用二氧化氮作为光媒介和分子氧作为终端氧化剂将甲烷转化为甲醇的方法(已发表于Inorg. Chem.)。该过程可以实现17%的甲烷转化率,同时对CH3ONO2的选择性高达78%。HCl催化光硝化途径的理论与实验结果吻合。 Activation of methane and its conversion to added-value products is an important topic which requires chemical solutions with high yields and selectivity. In our recent collaborative study, we present the oxidation of methane to methanol using nitrogen dioxide as a photo-mediator and molecular oxygen as the terminal oxidant. This process achieves the methane conversion of 17% while the selectivity towards CH3ONO2 is as high as 78%. Joined experimental and theoretical investigation support the HCl-catalyzed photo-nitration pathway. 提出这个方法的动机是为了解决选择性转化高惰性甲烷的挑战。甲烷在大气中通过涉及NO2的复杂光反应自然发生活化,但NO2在大规模甲烷转化中的潜力尚未实现完全探索。 The motivation behind the presented proposal is to address the challenges in selectively converting methane which is highly inert. Methane activation occurs naturally in the atmosphere through complex photoreactions involving NO2, but the potential of NO2 in large-scale methane conversion is not fully explored. 我们计划建立一个化学循环实现光驱动NO2介导的CH4转化,将其转化为CH3OH,其中CH3ONO2作为气体中间体。该策略可以避免过氧化,而且可以通过硝酸甲酯的水解获得甲醇这个重要的平台化学品。 The plan is to construct a chemical looping for the photo-driven NO2-mediated CH4 conversion to CH3OH via CH3ONO2 as a gas intermediate. This strategy can prevent over-oxidation and obtain methanol as an important platform chemical through the hydrolysis of methyl nitrate. 激发态NO2具有活化烷烃的能力,这已被它在低温(-10°C)下与乙烷的光硝化反应所证实。然而,NO2和甲烷之间的反应直到180°C才开始发生,在这个温度下甲烷主要被转化为COx(该现象与理论计算结论一致)。我们发现了CH4与基态2A1态下NO2反应的三条不同途径(见下图)。所有这些途径的吉布斯自由能垒都在37-44 kcal/mol的范围内,比低温(-10°C)反应的能垒高的多。我们没有找到任何CH4与激发态2B2态下NO2反应的反应路径。 Excited NO2 has demonstrated the ability to activate alkanes, as evidenced by the successful photo-nitration of ethane at a low temperature of -10°C. However, the reaction between NO2 and CH4 does not take place until 180°C, at which temperature the methane is converted to COx as the main products. Theoretical investigations also support this experimental phenomenon. We found three different pathways for the reaction of CH4 with NO2 in the ground, 2A1 state (see figure below). Nevertheless, all of them have the Gibbs free energy barriers of 37-44 kcal/mol, which are too high for a reaction to take place at a low temperature of -10°C. We could not locate any reaction paths for CH4 and NO2 in the excited, 2B2 state. 幸运的是,我们发现HCl可以通过氢原子转移(HAT)反应催化甲烷的光硝化。理论研究表明,HCl可以以两种不同的方式与NO2(2A1)反应,其自由能垒(26-33 kcal/mol)比CH4与NO2(2A1)反应自由能垒低,但仍然没有低到可在-10°C下轻易进行。在NO2分子被450 nm LED照射激发后,HCl可以与NO2(2B2)发生反应,其自由能垒仅为20.4 kcal/mol(见下图)。因此,在可见光的帮助下, HCl与NO2发生氢提取反应产生Cl•自由基。在线质谱(MS)证实了Cl•自由基的存在,此外,光激发Al(NO3)/AlCl3混合物后大量的HONO被检测到。 Fortunately, we found that HCl can catalyze methane photo-nitration via relay hydrogen atom-transfer (HAT) reactions. Theoretical investigations show that HCl can react with NO2 (2A1) in two different ways with lower free energy barriers (26–33 kcal/mol) than CH4 but the energy barriers are still not low enough to make these reactions happen easily at -10°C. After NO2 molecules are excited by the 450 nm LED illumination, HCl can react with NO2 (2B2) species with the low 20.4 kcal/mol free energy barrier (see figure below). Thus, the free Cl• radical can be readily generated from the HCl molecule via the H-abstraction by NO2 with the assistance of visible light. The existence of Cl• radical is supported by the online MS and a substantial amount of HONO is also detected after photoexciting a mixture of Al(NO3)/AlCl3. 随后,Cl•自由基与CH4反应,重新生成HCl并产生CH3•自由基。CH4与Cl•自由基之间的氢原子转移反应(HAT)的能垒(约为1.67 kcal/mol)要比CH4与NO2之间反应的能垒(37–44 kcal/mol)低得多。接着,通过CH3•自由基与O2、NO2、NO之间的反应,可以在相对较低的温度下选择性生成CH3ONO2(可以水解生成CH3OH)。 The Cl• radical can then react with CH4 to regenerate HCl and produce CH3• radical. The HAT between CH4 and Cl• radical has a much lower barrier (about 1.67 kcal/mol) than that between CH4 and NO2 (37–44 kcal/mol). Then, CH3ONO2 can be selectively produced at a relatively low temperature via the reaction between CH3• radical and O2, NO2, NO. The produced CH3ONO2 can be hydrolyzed in water to produce CH3OH. 上述展示的催化循环的简明表示概括了提出的机制。该循环始于可见光激发加热硝酸铝Al(NO3)3产生的NO2;随后,激发态NO2与CH4和O2发生反应,产生硝酸甲酯(CH3ONO2);硝酸甲酯继而水解生成甲醇(CH3OH)。这个过程所生成的硝酸(HNO3)和硝酸根离子(NO3−),高效地循环回到Al(NO3)3。在甲烷的光硝化过程中,HCl作为催化剂起到促进氢原子转移(HAT)反应的作用。 这项工作为烷烃升级领域打开了新的机遇。我们所提出的策略可以激发对其它烷烃转化和官能化新途径的探索。 This work has opened up new opportunities in the field of alkane upgrading. The strategy proposed here can inspire the exploration of new pathways for the conversion and functionalization of other alkanes. 论文信息 Xuefeng He, Lina Zhang, Jiawei Chen, Huichong Liu, Yuming Su, Han Li, Yonghua Cao, Pavlo O. Dral*, Cheng Wang*. Photo-Driven Aerobic Methane Nitration. Inorg. Chem. 2023, 62, 10343–10350. DOI: 10.1021/acs.inorgchem.3c01210.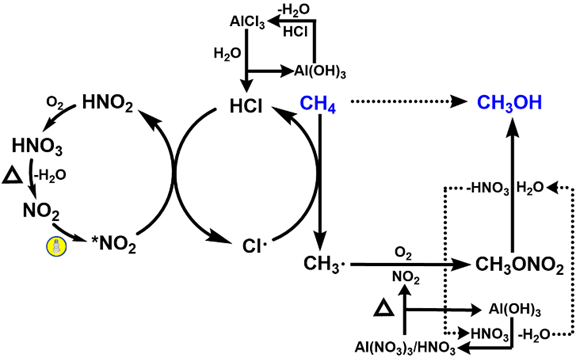
The concise representation of the catalytic loop shown at the top summarizes the proposed mechanism. It starts with visible light exciting NO2 which is generated by heating aluminum nitrate Al(NO3)3. The excited NO2 then reacts with CH4 and O2, resulting in the production of methyl nitrate (CH3ONO2). This methyl nitrate is subsequently hydrolyzed to produce CH3OH. During the process, nitric acid (HNO3) and nitrate (NO3−) are generated and efficiently recycled back to Al(NO3)3. In the photo-nitration process of methane, HCl acts as a catalyst facilitating relay hydrogen atom transfer (HAT) reactions.