Chem. Eur. J.: Application of Valence Bond Theory to Captodative Effect in Diboron Molecule (CAAC)2B2(SH)2
Published Time: 2024-08-05 15:55:05
Editor's
message: This work showcases the application of the block localized wave
function (BLW) method and the valence bond self-consistent field (VBSCF) method
within the XMVB@XACS software package by Huaiyu Zhang at Hebei Normal
University, Yirong Mo at the University of North Carolina at Greensboro and coworkers.
The authors investigated the push-pull effect in the diboron molecule (CAAC)2B2(SH)2
caused by the co-planarity of the thiol groups. The study is valuable in
guiding the design and performance regulation of diboron compounds.

Due to the electron-deficient nature of boron atom, boron-containing compounds often exhibit unique electronic structures and chemical properties, with wide potential applications in organic synthesis and catalysis. In recent years, unsaturated boron compounds have attracted significant attention from chemists. Braunschweig et al. found that the cyclic (alkyl)(amino) carbenes (CAACs) stabilized diboron molecules (CAAC)2B2(SR)2 host unpaired electrons and exist in the 90°-twisted diradical form, while other analogues, such as N-heterocyclic carbenes (NHCs), stabilized diboron molecules prefer a conventional B=B double bond. A thorough understanding of the effects of carbenes and substituents on diboron compounds is of great significance for guiding the synthesis and performance regulation of diboron compounds.
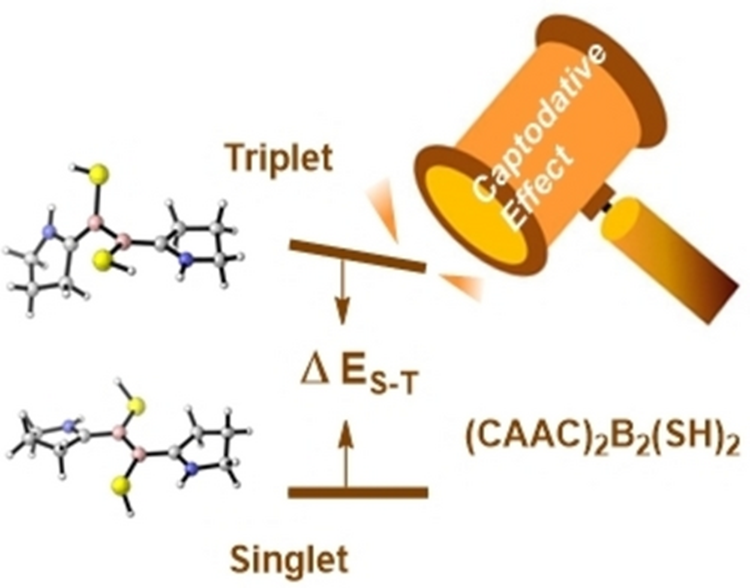
In this work,
the authors employed the block localized wave function (BLW) method and the
valence bond self-consistent field (VBSCF) methods to progressively localize
electrons in combination with geometric changes and energy analyses and
discussed the push-pull effect caused by the co-planarity of the thiol groups
and its impact on the singlet-triplet energy gap of the diboron molecule (CAAC)2B2(SH)2.
The computations demonstrated a significant reduction in the singlet-triplet
energy gap of (CAAC)2B2(SH)2 due to the
co-planarity of the thiol groups. Specifically, the thiol groups serve as a
π-electron-donating group, while the CAAC ligand acts as a π-electron-accepting
group. Consequently, the co-planarity of the thiol groups leads to a π-electron
push-pull effect in the diboron molecule (CAAC)2B2(SH)2.
Based on the geometric characteristics and energy changes, it is concluded that
the repulsion from the lone pair electrons in the thiol groups enhances the
delocalization effect between the B=B center and the CAAC carbene, while the
delocalization effect of the lone pair electrons in the thiol groups leads to a
further elongation of the B-B bond. Furthermore, VBSCF calculations reveal that
the push-pull channel in (CAAC)2B2(SH)2
primarily exists within the two (CAAC)B(SH) fragments.
This study demonstrates that the co-planarity of the thiol groups and the resulting π-electron push-pull effect can significantly reduce the singlet-triplet energy gap of (CAAC)2B2(SH)2, providing important guidance for the design and performance regulation of diboron compounds.
Paper:
Captodative Effect Facilitates the Excitation in Diboron Molecule (CAAC)2B2(SH)2
Huaiyu Zhang,* Yating Wang, Qingrui Lu, Jinshuai Song, Yandong Duan, Yanli
Zeng, and Yirong Mo*
DOI: 10.1002/chem.202203817