Chem. Eur. J.: Valence bond theory study of the impact of solvents on the strength of coordinate covalent and ionic bonds
Published Time: 2024-08-23 14:54:05
Editor's message: This work showcases the application of the block localized wave function (BLW) method to explain the impact of solvents on the strength of coordinate covalent and ionic bonds by Changwei Wang at Shaanxi Normal University, Yirong Mo at the University of North Carolina at Greensboro and coworkers. The study also employed the XMVB@XACS program to perform valence bond self-consistent field (VBSCF) calculations to analyze the potential energy surface (PES), and used the XEDA@XACS program to carry out energy decomposition analysis (EDA) of the PES with explicit solvent models.

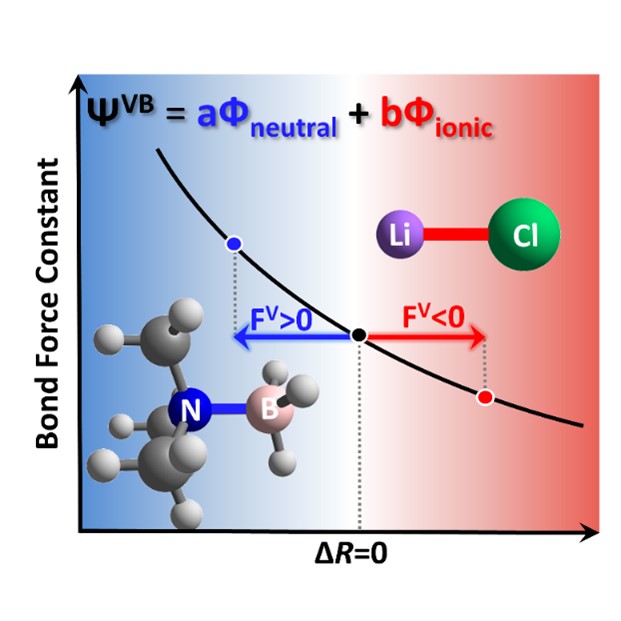
The influence of solutions on the strength of chemical bonds is a fundamental chemical issue. In this regard, the enhancement of the N-B bond in Me3NBH3 molecules induced by polar solvents and the weakening of LiCl ionic bond are representative examples. This work takes Me3NBH3 and LiCl as examples, respectively, and applies PES analysis methods to explain the solvent's impact on bond lengths and vibrational frequencies from two aspects: the force exerted on chemical bonds by the solvent environment and its effect on force constants. Furthermore, this work applies ab initio VB theory and its minimalist variant, the block-localized wavefunction (BLW) method, to analyze in detail the response of bond strength to solutions from a perspective familiar to chemists.
This work
developed a PES analysis that can intuitively elucidate the impact of the
solvent environment on bond lengths and vibrational frequencies from the
perspectives of energy, force, and force constant. The energy analysis results
indicate that the shortening of the N-B bond and the elongation of Li-Cl can
enhance solvent-solute interactions. This trend can be intuitively demonstrated
by the gradient of solvent-solute interactions with bond length changes, that
is, the force exerted by the solvent on chemical bonds: the N-B bond and Li-Cl
bond are subject to positive and negative solvent-solute interaction forces,
respectively, and thus need to shorten or elongate to achieve equilibrium. In
both systems, the inherent energy of the solute determines the trend of changes
in the force constant: the second derivative of the curve of the force constant
with bond length decreases as the bond lengthens. Consequently, the contraction
of the N-B bond increases its force constant, while the elongation of the Li-Cl
bond decreases its force constant. Additionally, this work used the ABCluster
program to conduct a PES analysis of the explicit solvent model, confirming the
above explanation based on force and force constant. Moreover, this work
applied the generalized Kohn–Sham EDA (GKS-EDA) method to analyze the PES of
the explicit solvent model, revealing that the direction of the force exerted
by the solvent environment on the system is determined by electrostatic
interactions.
The trend of the solute molecule's dipole moment with respect to bond length variation determines its response to the solvent environment. Specifically, the dipole of Me3NBH3 increases as the bond length shortens, thus tending to shorten the N-B bond in solution to enhance solvent-solute interactions. In contrast, the dipole of LiCl increases with the elongation of the bond length, thereby enhancing solvent-solute interactions through bond elongation in polar solutions. The aforementioned dipole moment variation with bond length can be explained by VB theory. The VB wavefunction is a linear combination of covalent and ionic structures. In both systems, the ionic structures have the characteristic of charge separation, and their dipole moments increase with the increase of bond length. On the contrary, the dipole of the covalent structure increases with the shortening of the bond length, since the shortening of the bond length can enhance polarization and increase the dipole. The trend of the dipole variation with bond length for Me3NBH3 and LiCl is determined by the most stable VB structure. The analysis of the dipole moments indicates that the ionic structures of Me3NBH3 and LiCl can enhance solvent-solute interactions by elongating the bond length in polar solutions, exhibiting a redshift in the stretching vibration frequency, while the covalent structures shorten the bond length to increase the dipole and solvent-solute interactions, exhibiting a blueshift phenomenon. Ultimately, the strength and vibrational frequency of coordinate covalent and ionic bonds in response to the solvent environment are determined by the most stable Lewis structure. The above inferences are confirmed by PES analysis based on ab initio VB theory, and geometry optimization and frequency calculations using the BLW method.
Article Messages:
Xinru Peng, Jiayao Li, Yakun Fan, Xubin Wang, Shiwei Yin, Changwei Wang,* Yirong Mo*
DOI: 10.1002/chem.202402008