ACS Catal: Study on Unsymmetric N‑Aryl Substituent Effects
Published Time: 2024-02-02 14:45:16
Editor's note: This research highlight showcases the application of GKS-EDA method from the XEDA@XACS software package in elucidating the role of non-covalent interactions in chemical reactions. It explains the impact of non-covalent interactions on the chemo- or stereoselectivity of chemical reactions.

The energy decomposition analysis method can be employed not only for the quantitative analysis of chemical bonds and molecular interactions but also for the investigation of chemical reaction mechanisms. In 2020, our research group utilized the GKS-EDA method to study the activation of the C-Br bond in diphenyl bromomethane, revealing the role oflone pair…π interactions in the activation of carbon-halogen bonds (ACS Omega 2020, 5, 34, 21862–21872).
In 2023, external research group conducted a series of theoretical studies on chemical reactions using the GKS-EDA method. Some of these studies include:
Professor Yanli Zeng’s group at
Hebei Normal University employed the GKS-EDA method to investigate the
catalytic bromination reaction by tellurium cations involving sulfur bonds:
Chalcogen Bond Catalysis withTelluronium Cations for Bromination Reaction: Importance of Electrostatic andPolarization Effects (Chem.Eur.J. 2023,29,e202302749)
The research team led by Professor
Ganglong Cui at Beijing Normal University conducted work on the photocatalysis
of carbon dioxide:
Photocatalytic Reduction of CO2 toHCOOH and CO by a Phosphine‐Bipyridine‐Phosphine Ir(III) Catalyst:Photophysics, Nonadiabatic Effects, Mechanism, and Selectivity (Angew. Chem. Int. Ed. 2023, e202315300)
The research team led by Professor
Chun-Yu Ho at Southern University of Science and Technology conducted a study
on the asymmetric aryl substitution effect:
Unsymmetric N-Aryl SubstituentEffects on Chiral NHC-Cu: Enantioselectivity and Reactivity Enhancement byOrtho-H and Syn-Configuration (ACS Catal. 2023, 13, 1, 407–421)
Here is a brief introduction to the work by Chun-Yu Ho et al. Since the early attempts by Hosomi and Miyaura, researchers have synthesized catalysts for the synthesis of boron compounds for tert-carbon centers. However, achieving high enantioselectivity in the synthesis of boronated products with chiral quaternary carbon centers remains challenging. The authors addressed this synthetic challenge through experimental and theoretical studies. They designed a catalyst based on nitrogen heterocyclic carbene-copper complexes. This catalyst demonstrated high yields and enantioselectivity for the boronation of α, β-unsaturated ketones with various substituents, including aryl, heteroaryl, and alkyl groups, providing boronated products with chiral quaternary carbon centers.
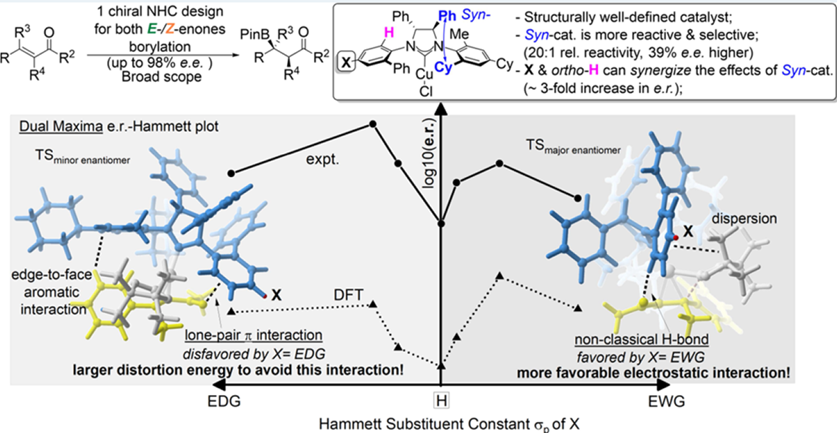
To elucidate the phenomena, this paper delves into the study of the role of non-covalent interactions in the enantioselectivity of catalysts. The team decomposed the activation energy of the boronation reaction into deformation energy between the catalyst and substrate molecules, deformation energy of substituted enone, and through-space, through-bond interaction energies between the enone and the ligand.
The GKS-EDA method was employed to analyze the interactions between enones and ligands in various transition state configurations using different ligands. The study explored atypical hydrogen bonding interactions between the enone substrate and the ligand, as well as the impact of lone pair electron-π interactions on the stability of various transition state configurations.
The quantitative analysis results from GKS-EDA elucidated the nature of various non-bonding interactions and revealed the patterns of changes in hydrogen bonding and lone pair…π interactions. Based on these patterns, the authors explained the differences in the stability of different transition states, providing an interpretation for the experimental results.
Reference:
Jianwei Xi, Elvis Wang Hei Ng, and Chun-Yu Ho*
XACS Research Highlights
XACS Research Highlights are posts about examples of research carried out using the XACS platform. We welcome the users to submit their blog posts to us at xacs@xmu.edu.cn or directly to one of the PIs and we will highlight your research on the official XACS channels and social accounts. We reserve the right to small editorial changes for clarity and corrections.