Spectroscopy
It is available for MLatom to simulate different kinds of spectra. Here we will show how to use MLatom for power spectra and IR spectra.
Power spectra
MD trajectory can be used to generate the power spectra. Here we choose ethanol as example.
To generate the power spectrum of ethanol in command line, we should prepare the input file
ethanol_ps.inp and the auxiliary file ethanol_traj.h5
documenting the molecular dynamics trajectory of ethanol.
# ethanol_ps.inp
MD2vibr # Calculate vibrational spectrum from MD
trajH5MDin=ethanol_traj.h5 # Read MD trajectory
output=ps # Calculate power spectrum
Run it.
mlatom ethanol_ps.inp
Import a specific module in Python so that the result will appear in .png format.
from IPython.display import Image
Image(filename='ps.png')
IR spectra
MD trajectory can also be used to generate IR spectra. Similar with the power spectra, in command line we need to
prepare the input file ethanol_ir.inp and the MD trajectory ethanol_traj.h5.
# ethanol_ir.inp
MD2vibr # Calculate vibrational spectrum from MD
trajH5MDin=ethanol_traj.h5 # Read MD trajectory
output=ir # Calculate infrared spectrum
Run the input file.
mlatom ethanol_ir.inp
Import a specific module in Python so that the result will appear in .png format.
from IPython.display import Image
Image(filename='ir.png')
There is the code for simulating IR spectra in Python.
You can see the spectrum (ir.png) in your folder after the computation is finished:
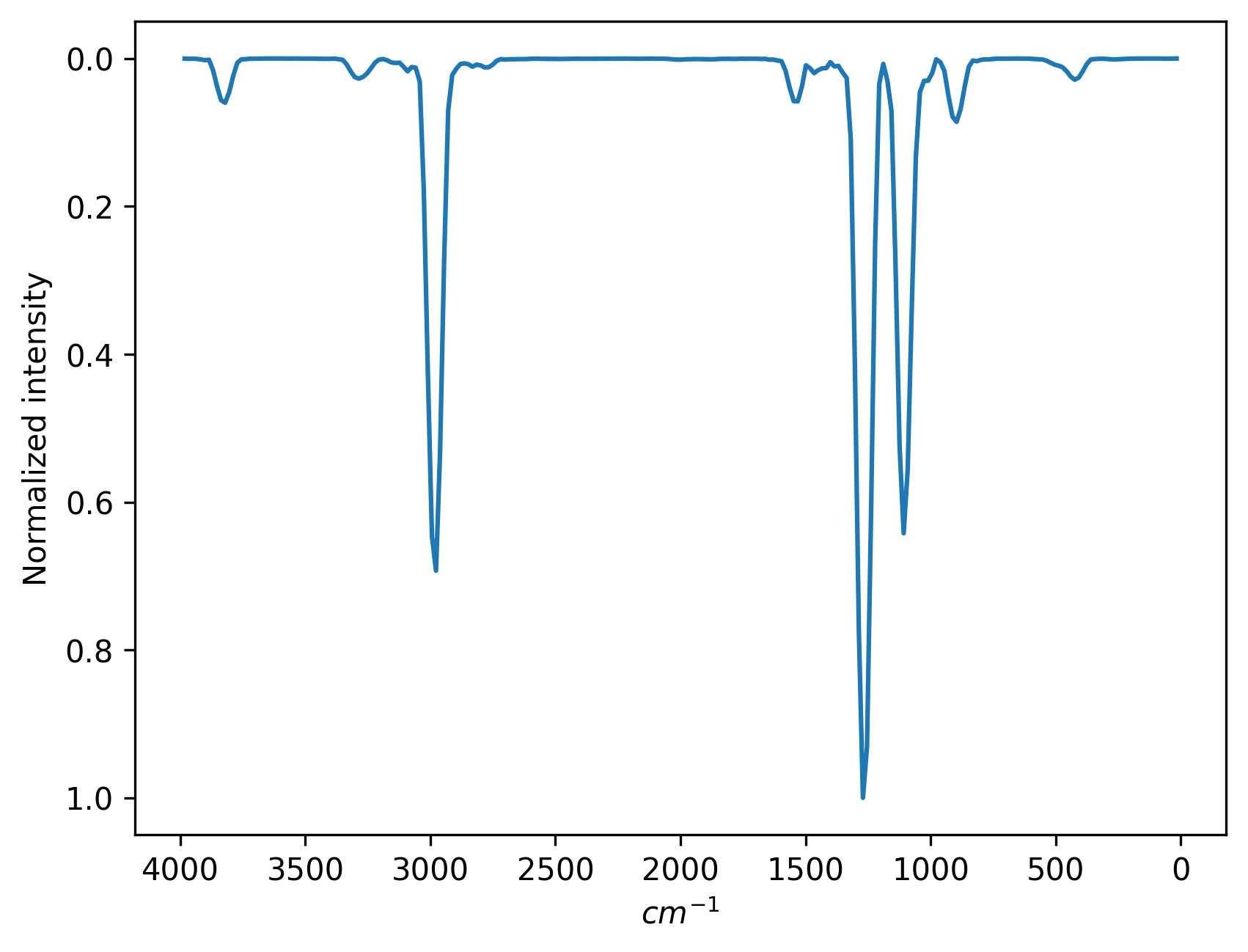
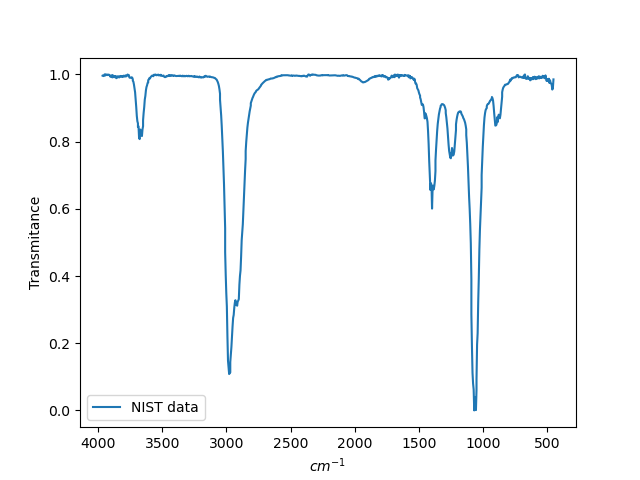
Below, for comparison, is the spectrum from the NIST database:
import mlatom as ml
traj = ml.data.molecular_trajectory()
traj.load('ethanol_traj.h5',format='h5md')
dt = traj.steps[1].time - traj.steps[0].time
moldb = ml.data.molecular_database()
moldb.molecules = [each.molecule for each in traj.steps]
vib = ml.vibrational_spectrum(molecular_database=moldb, dt=dt)
vib.plot_infrared_spectrum(filename='ir_api.png')
from IPython.display import Image
Image(filename='ir_api.png')
UV/vis absorption spectra
Machine learning can be used to greatly accelerate the calculation of precise UV/vis absorption spectra via nuclear ensemble approach (NEA) which typically requires many hundreds or thousands of expensive quantum mechanical calculations of excited states. MLatom supports such ML-accelerated NEA calculations as described in our paper.
The following example is from our book chapter.
MLatom input file:
cross-section
Nexcitations=30
plotQCNEA
plotQCSPC
deltaQCNEA=0.05
These calculations require many data files (reference excitation energies at TDDFT level).
These data files are zipped and should be uploaded as a zip archive to the cloud computing as auxiliary file.
Calculations can take more than 5 min. MLatom automatically determines the minimum required number of training points, in this case it needed 200 points for precise spectrum. In the output file you can find that it took 4 iterations to converge:
==========================================================================================
run ML-NEA iteratively for spectrum generation ( ML_train_iter ) started at Wed Dec 1 12:00:19 2021 CST
ML-NEA iteration 1: train_number = 50; RMSE_geom = 0.06717941145022376; rRMSE = 1.0
ML-NEA iteration 2: train_number = 100; RMSE_geom = 0.09043318436728051; rRMSE = 0.25713761026721255
ML-NEA iteration 3: train_number = 150; RMSE_geom = 0.06411060145373663; rRMSE = 0.410580813729204
ML-NEA iteration 4: train_number = 200; RMSE_geom = 0.0695737045717655; rRMSE = 0.07852252732055763
ML-NEA iteration ended after 4 iteration!
run ML-NEA iteratively for spectrum generation ( ML_train_iter ) finished at Wed Dec 1 12:08:01 2021 CST |||| total spent 462.02 sec
==========================================================================================
After the calculations finished, the spectra are plotted to plot.png file in the cross-section sub-directory. It should look like:
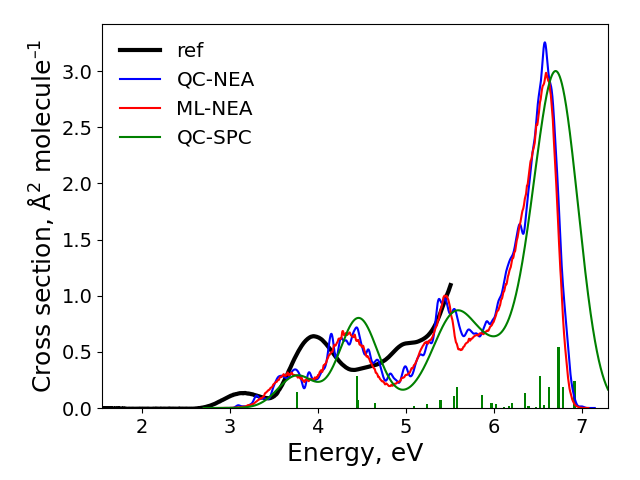
The final result: ‘ref’ is the experimental spectrum, QC-NEA – spectrum calculated with quantum chemical approach on 200 points in ensemble, ML-NEA – machine learning spectrum generated with 200 points in the training set and 50k points in ensemble, QC-SPC – spectrum generated with single-point convolution.
Compare obtained spectra and answer the following questions:
What are the problems with the QC-NEA spectrum?
What are the problems with the QC-SPC spectrum?
How to improve accuracy of the ML-NEA spectrum?
Two-photon absorption cross sections
Two-photon absorption (TPA) is an important physical phenomenon which can be exploited in many different applications like upconverted laser. MLatom implements machine learning method predicting TPA cross section for a new molecule just by providing its SMILES (see this paper in Adv. Sci. for details).
Here we show how to calculate TPA cross section for RHODAMINE 6G and RHODAMINE 123 molecules with MLatom input file mltpa.inp:
MLTPA
SMILESfile=Smiles.csv
auxfile=_aux.txt
This input requires Smiles.csv file with SMILES of molecules:
CCNC1=CC2=C(C=C1C)C(=C3C=C(C(=[NH+]CC)C=C3O2)C)C4=CC=CC=C4C(=O)OCC.[Cl-]
COC(=O)C1=CC=CC=C1C2=C3C=CC(=N)C=C3OC4=C2C=CC(=C4)N.Cl
and optional _aux.txt, which defines the wavelength_lowbound, wavelength_upbound, and Et30 for making predicitons:
600,850,55.4
600,600,33.9
After you prepared your input files mltpa.inp, Smiles.csv, and _aux.txt, you can run MLatom as usual.
After the calculations finish, the predicted TPA cross section values are saved in two files for two molecules: tpa1.txt and tpa2.txt. For our examples, they look like:
wavelength,predicted_sigma (GM)
600.0,285.19455
610.0,297.71707
620.0,284.11694
......
810.0,121.51988
820.0,116.537994
830.0,118.04909
840.0,103.65925
850.0,113.72374
and
wavelength,predicted_sigma (GM)
600.0,138.2346